SpiroConnect® - Product Information
Precision Spirometry for all Applications
SpiroConnect® is easy to use, extremely stable, reliable and accurate at all flows. It connects conveniently via latest Bluetooth technology to the PC, lap top computer or a mobile android device.



Turbine spirometers are characterised by extremely stable calibration1,2,3. In contrast other types of spirometer transducer may be sensitive to environmental factors and contamination4.
Its performance has been independently tested in a study aiming to establish the accuracy of spirometry measurements produced by SpiroConnect® in comparison to those obtained from a pitot-type spirometer and no clinically significant differences in the FEV1 or VC measurements occurred in the two devices.5

SpiroConnect® with its patented6 vertical turbine technology and Bluetooth connectivity combines all known advantages of existing turbine spirometers with technological innovations to deliver precision spirometry for diagnoses and management of Asthma and COPD.

SpiroConnect® is the only turbine spirometer achieving the low flow sensitivity required by the ATS/ERS guidelines of 0.025l/sec - particularly important for the diagnosis and monitoring of COPD, a condition characterised by an extended period of low flow in the spirometry manoeuvre.
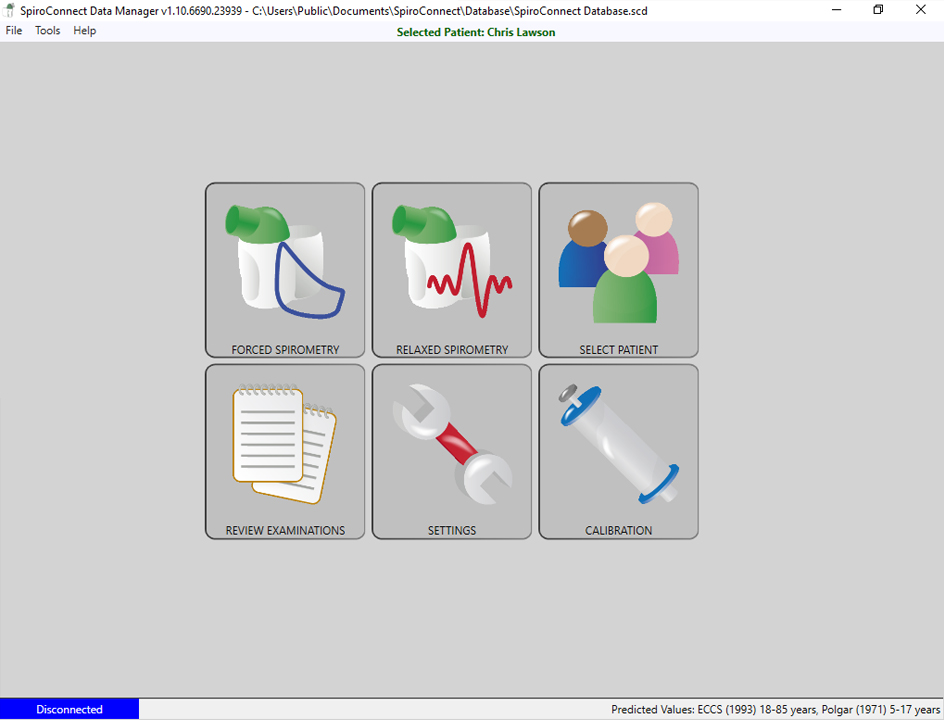
SpiroConnect® Data Manager is a user friendly and intuitive PC software with functional icons and an online operating manual. It allows for a real time graphical display providing immediate patient and operator feedback and quality control, data storage, test and calibration-check report printout. It supports the following predicted value data sets: GLI, ECCS, Polgar, Zapletal and Austrian-Forche. The acceptance criteria can be selected from: ARTP, ATS/ERS or BTS. In the UK, SpiroConnect® Data Manager has been fully integrated with EMIS, TPP SystmOne and Vision.
An on-screen Child Incentive animation assists in performing reliable spirometry tests by trained personnel even in preschool children.
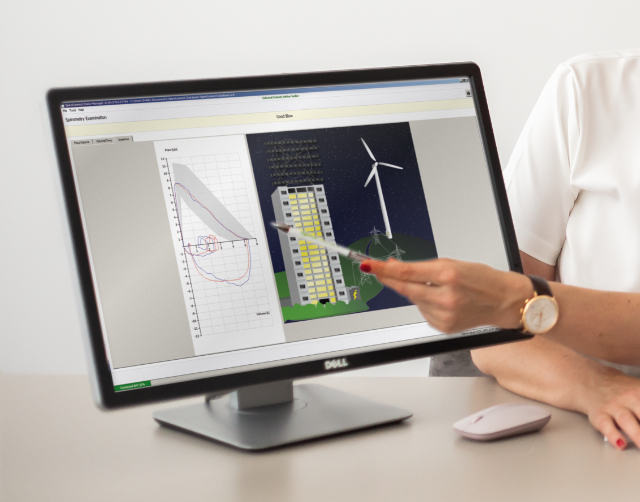
SpiroConnect® Mobile App allows users to collect spirometry data on an Android tablet or mobile phone, and then upload the results to the patient database on the PC. Forced spirometry, Relaxed spirometry, post bronchodilator testing, calibration, examination review and patient data entry are all supported.

The simple infection prevention procedure involves immersing all parts coming in the contact with patient's exhaled air in a cold sterilising solution or the use of standard pulmonary filters or one-way valve mouthpieces.

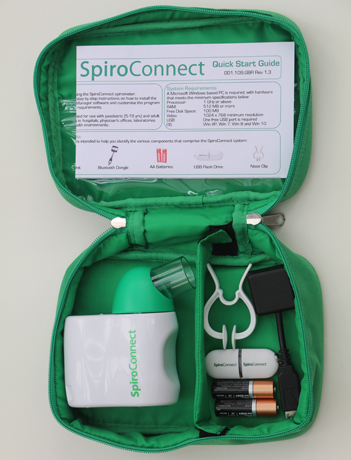
SpiroConnect® spirometer comes in a soft carry pouch complete with SpiroConnect® Data Manager software on USB flash drive memory stick, Bluetooth dongle, nose clip, 2 Alkaline AA (LR6) batteries, quick start guide and the calibration certificate.
Specifications:
| Transducer Type | Bi-directional high sensitivity vertical turbine |
| Measurements | VC, FEV0.75, FEV1, FEV3, FEV6, FVC, PEF, FEF25 (MEF75), FEF50 (MEF50), FEF75 (MEF25) FEF25-75 (MMEF), FIV1, FIVC, PIF, FIF25 (MIF75) FIF50 (MIF50), FIF75 (MIF25), MET25-75, FET, EVC, IVC, IC, VT (TV), Ti, Te, IRV, ERV, Vext, FEV0.75/VC, FEV0.75/FVC, FEV1/VC, FEV1/FVC (FER) , FEV3/VC, FEV3/FVC, FEV0.75/FEV6, FEV1/FEV6, FEF50/VC, FEF50/FVC, MMEF/FVC (FEF25-75/FVC) , FIV1/FIVC (FIR), R50 (FEF50/FIF50), Ti/Ttot, VT/Ti (TV/Ti), Lung Age, MVVind (FEV1x0.35) |
| Accuracy | To ISO 26782 and ATS 2005 standards |
| Sensitivity | Better than 0.025l/s |
| Power Supply | 2 x AA size Alkaline primary cells |
Operating Current |
110 mA peak |
| Battery Life | Alkaline cells, greater than 100 measurement cycles |
| Dimensions | 55mm(W) x 100mm(D) x 110mm(H) |
| Weight (including batteries) |
200g |
| Operating Conditions | 10°C to 38°C, 15% to 95% RH, non-condensing, altitude up to 3,000m |
| Transport and Storage Conditions |
-20°C to 70°C, 15% to 95% RH, non-condensing |
| Lifetime | 5 years |
| Flow Range | 0- 14l/s |
| Volume Range | 0 to 8l |
| PC Software Requirements | A Microsoft Windows based PC is required, with hardware that meets the minimum specifications below: Processor: 1 GHz or above RAM: 512 MB or more Free Disk Space: 100 MB Video: 1024 x 768 minimum resolution USB: One free USB port is required OS: Win XP, Win 7, Win 8 and Win 10 |
| Android App Software Requirements | Android tablets and phones with an Android 4.2 or greater and that have integral Bluetooth connectivity and a screen resolution of at least 480 x 800 |

Images







